Product Specifications are a North Star for MMS Manufacturing
By Jarno de Lange, Associate Director, Supply Chain at Vitamin Angels

Whether it’s delivering Vitamin A supplementation, deworming tablets, or prenatal multiple micronutrient supplements (MMS), Vitamin Angels has a core tenet of quality. All the product-based interventions that we provide must pass rigorous standards. We consistently aim for and reach the highest internationally recognized quality standards achievable. Women and children deserve that and more; it’s an integral part of the ethos of Vitamin Angels.
As the supply chain director, a critical part of my job is to ensure that our quality protocol for manufactured products is applied. We engage with manufacturers through stringent prequalification and quality control processes (including independent lab testing) to ensure quality is built into the nutrition products we procure. Product specifications, documents that outline the requirements for a product, serve as our “north star” – critical for securing high-quality products, consistently, no matter which manufacturer we use or their location.
I’ll give you the perfect example of why a product specification is so important. Multiple Micronutrient Supplements (MMS) – a proven, evidence-based prenatal vitamin and mineral supplement for pregnant women – consists of 15 vitamins and minerals. MMS is a complex and shelf-life sensitive product with many ingredients, and manufacturers can approach development and production many ways. Having a “north star” in the form of a product specification reduces the likelihood of substandard products and takes away any guesswork about the product we are looking for.
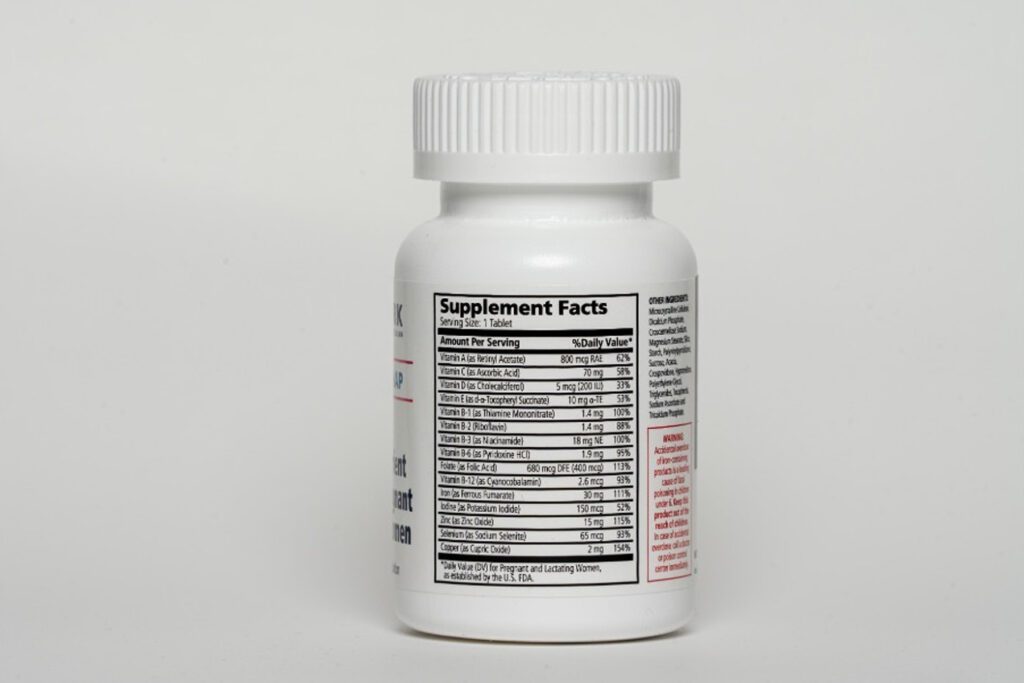
This year, the Annals of the New York Academy of Sciences published the Expert Consensus on an Open-Access UNIMMAP MMS Product Specification, 2024 revision describing the requirements for producing the United Nations International Multiple Micronutrient Antenatal Preparation (UNIMMAP) formulation of MMS. Setting clear expectations around the product standards, this product specification helps us and our manufacturers to reach agreement, in advance, on the nature of the product being purchased.
“Product specifications help us to harmonize the product development and manufacturing process with all manufacturers globally, so we’re all meeting the same expectations and ultimately creating an effective and high-quality product.”
– Jarno de Lange, Vitamin Angels
This product specification is the result of a collaborative effort among the Vitamin Angels, Kirk Humanitarian, and the Multiple Micronutrient Supplementation in Pregnancy Technical Advisory Group (MMS TAG) hosted by the Micronutrient Forum (MNF), and aims to support production of a low-cost, high-quality MMS product for public and private markets.
Early adoption of these specifications by manufacturers is yielding incredible results, including:
- An MMS product of higher and standardized quality. Purchasers and manufacturers both know up front what the requirements are for ingredients, food additives, processing aids, stability testing, storage, transport, and how to meet all regulatory certifications. The result is a UNIMMAP MMS product that is consistent in quality and interchangeable, regardless of what manufacturer it was procured from.
- Clearer understandings of product and market expectations. Including the product specification in purchase agreements helps purchasers, like Vitamin Angels, to support larger market activation. For example, standardization of MMS production in Indonesia, India, Pakistan, and Nigeria opens the possibility for them to serve as regional supply hubs for other countries without the manufacturing base needed to support MMS production.
- Growing global capacity for MMS production. The public availability of the product specifications (open access) is driving broader interest from national governments in the production of UNIMMAP MMS. UNICEF, one of the world’s largest providers of humanitarian supplies, acknowledges that the product specification aligns with their standards for UNIMMAP MMS, which unlocks further potential to grow manufacturing capacity. The product specification is also enabling manufacturers and purchasers to address a major barrier before starting development – determining whether MMS is classified as a dietary supplement or a medicine in the country of use.
I’m proud to have contributed to this latest milestone in Vitamin Angels’ push for better maternal nutrition across the globe. For three decades, Vitamin Angels has collaborated with partners to improve access to evidence-based interventions for those who need them most. In the 1990s, Vitamin Angels began to deliver high-dose vitamin A capsules to young children in low- and middle-income countries. Those efforts gradually expanded to other evidence-based nutrition interventions, including UNIMMAP MMS, and technical services to introduce or strengthen delivery of those interventions by national governments.
Today, we collaborate with over 1,200 partners, including national governments and non-governmental organizations, to provide nutrition products and technical services to those who need them. A common thread across the products we provide is a commitment to delivering high-quality products consistent with internationally recognized standards. The Expert Consensus on an Open-Access UNIMMAP MMS Product Specification is a testament to that commitment, and a powerful tool for shaping a marketplace for high-quality nutrition products to benefit pregnant women and their babies.
